Prime editing enables precise installation of genomic substitutions, insertions and deletions in living systems. Efficient in vitro and in vivo delivery of prime editing components, however, remains a challenge. Here we report prime editor engineered virus-like particles (PE-eVLPs) that deliver prime editor proteins, prime editing guide RNAs and nicking single guide RNAs as transient ribonucleoprotein complexes. We systematically engineered v3 and v3b PE-eVLPs with 65- to 170-fold higher editing efficiency in human cells compared to a PE-eVLP construct based on our previously reported base editor eVLP architecture. In two mouse models of genetic blindness, single injections of v3 PE-eVLPs resulted in therapeutically relevant levels of prime editing in the retina, protein expression restoration and partial visual function rescue. Optimized PE-eVLPs support transient in vivo delivery of prime editor ribonucleoproteins, enhancing the potential safety of prime editing by reducing off-target editing and obviating the possibility of oncogenic transgene integration. Delivery of prime editors in vivo is improved using virus-like particles.
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account
 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
A study in mice reports a CRISPR/Cas9 gene-editing delivery system, capable of bypassing the blood-brain-barrier and modulating neuronal receptor pathways, to treat chronic anxiety.
BigField GEG Tech's insight:
A mouse study reports on a CRISPR/Cas9 gene-editing delivery system, capable of bypassing the blood-brain barrier and modulating neuronal receptor pathways, to treat chronic anxiety. Researchers targeted 5HT-2A, a serotonin receptor known to play a role in anxiety and depression. The authors used a vector based on an inactivated adeno-associated virus to deliver the vector via the nose. The vector delivers a guide RNA to the neurons. The guide RNA binds to the target receptor gene, HTR2A, which is then cleaved at a precise location by the Cas9 enzyme. Five weeks after intranasal administration of the vector and packaging, 75 mice were tested with standard behavioral tests measuring mouse anxiety. For example, anxious mice would choose to spend more time in dark areas and would tend to bury unfamiliar objects such as logs in sawdust rather than leave them alone. Treated mice spent 35.7% more time in light areas than controls and showed a 14.8% decrease in the number of logs buried compared to controls. Mice treated with the CRISPR package showed an 8.47-fold decrease in HTR2A expression in their brains, compared with control mice. According to the authors, non-invasive intranasal administration of CRISPR/Cas9 therapies may therefore help patients with treatment-resistant anxiety.
Irish-Indian company CyGenica is on a mission to solve the huge challenge surrounding targeted intracellular delivery of gene-editing and other therapies. In just five years since the company was founded, the first clinical trials for cancer are in sight. Here, CEO and CSO Dr Nusrat Sanghamitra PhD tells us about how a persona
BigField GEG Tech's insight:
CyGenica is an Irish-Indian company founded in 2017 that specializes in novel cargo delivery technologies. The company has developed a non-viral, non-toxic platform called GEENIE to develop solutions for in vitro and in vivo delivery of drugs, gene editing components such as CRISPR-Cas9, and antibiotics. The GEENIE platform can be thought of as a protein drill that pierces the cell membrane and brings its cargo with it. It is a 48 KDa protein that is introduced into cells by a non-endocytic mechanism that does not damage the cells. It delivers its cargo to the nucleus, which is very relevant for gene editing therapies. For each target organ, researchers design the GEENIE platform separately to make it "go" to the right place in the body. This strategy works in vitro in HER2+ breast cancer and in glioblastoma. For each of these targets, a specific construct that recognizes a unique receptor on the target cells has been developed. This strategy can be applied to any tissue type as long as a unique receptor has been identified. Moreover, the researchers have so far administered GEENIE to animals by intravenous injection, but because this protein is so stable, they want to try to develop an inhalation method for lung treatments in the future.
Gene editing has revolutionised research and is set to change the treatment landscape for genetic diseases, with >100 ongoing clinical trials across diverse disease areas. Here, medical doctor Silja Hansen, who is currently a PhD student at Aarhus University in Denmark, discusses the promise, challenges, and future of prime editing t
BigField GEG Tech's insight:
Inherited retinal diseases are characterized by dysfunction and degeneration of the photoreceptors and/or the retinal pigment epithelium, leading to progressive vision loss and blindness. After winning a grant of just over DKK 3 million (EUR 400,000) from the Velux Foundation, PhD student Silja Hansen MD aims to develop a new treatment based on prime editing to slow the progression of inherited retinal diseases. This editing has the potential to correct all kinds of point mutations as well as smaller deletions and insertions that cover many of the variants underlying inherited retinal diseases. This tool functions as an all-in-one system thanks to the pegRNA that directs the enzyme complex to the target site and as its own template because it does not require a double-strand break. One of the challenges of prime editing is to find a pegRNA that can create the desired editing and achieve high targeting efficiency. Another major challenge is the delivery of the gene editing cargo. Previous studies have explored the possibility of using a dual adeno-associated virus to provide a split master editor system. They would like to test this strategy but would also like to investigate different delivery options, including non-viral delivery methods.
Patients with collagen VI-related disorders experience progressive and debilitating disease. New research findings from Spain’s Institut de Recerca Sant Joan de Déu (IRSJD) provides new hope for these diseases, demonstrating the power of CRISPR-Cas9 to silence a dominant negative mutation in patient fibroblasts.
BigField GEG Tech's insight:
Collagen VI-related dystrophies (COL6-RD) are caused by mutations in the COL6A1, COL6A2 and COL6A3 genes that encode the alpha chains of collagen VI, a key component of the extracellular matrix. The prevalent pathogenic mutation in COL6A1 is a single nucleotide substitution, where a glycine is replaced by an arginine in the N-terminus of the triple helix domain which affects the folding of the resulting protein, hindering the association of tetramers to create the necessary collagen VI microfibrils. Moreover, there is currently no effective treatment available for COL6-RD. However, a recent paper demonstrates the use of CRISPR-Cas9 gene editing to mitigate the pathogenic effects of a dominant negative mutation in the COL6A1 gene. Nevertheless, an obvious hurdle for this type of therapy is the delivery of CRISPR-Cas9 reagents to fibroblasts in vivo; successful editing of somatic tissues such as muscle remains a significant challenge in the broader field. The research team hopes that recent advances in several areas of CRISPR delivery vehicles, including muscle adeno-associated trophic virus vectors and tissue-specific nanoparticles, may be the answer.
We designed a unique nanocapsule for efficient single CRISPR-Cas9 capsuling, noninvasive brain delivery and tumor cell targeting, demonstrating an effective and safe strategy for glioblastoma gen
BigField GEG Tech's insight:
Many drugs cannot cross the blood-brain barrier (BBB), which also applies to most CRISPR-carrying vehicles. So Bingyang Shi made chemically functional nanocapsules to penetrate the BBB, according to a study recently published in Science Advances. He essentially packaged unique CRISPR-Cas9/sgRNA complexes in a coating consisting of a thin polymeric shell made of positively charged guanidine acrylate cross-linked with N,N-bis(acryloyl)cystamine that contains biodegradable disulfide bonds. The shell was finally decorated with the peptide angiopep-2. While coating rather than encapsulation was chosen to maintain the particle size at approximately 30 nm and thus facilitate BBB penetration, the angiopep-2 peptide served two purposes. It is a ligand that binds to low-density lipoprotein receptor-related protein-1, which is most highly expressed on BBB endothelial cells and glioblastoma (GBM) cells. This will result in the concentration of the nanocapsules around the BBB, increasing their chance of penetration into the brain, where they will then be preferentially taken up by GBMs. However, more work is needed before these nanocapsules can emerge as a therapy for GBM patients.
Researchers utilized a CRISPR/Cas9 system to evaluate the usage of tRNA by deleting two tRNA genes from the genomes of hyper hepatocellular carcinoma and human near-haploid chronic myeloid leukemia cells.
BigField GEG Tech's insight:
In a recent study, a team of researchers used a CRISPR/Cas9 system to evaluate the use of tRNA by deleting two tRNA genes from the genomes of hyperhepatocellular carcinoma and human chronic myeloid quasi-haploid leukaemia cells. The authors discovered numerous unexpected genomic changes at the target region using an improved droplet-based target enrichment approach followed by Oxford Nanopore Technology long-read sequencing. The method used in this study demonstrates that CRISPR/Cas9 can lead to the integration of endogenous and exogenous DNA fragments and also produce local inversions, duplications and insertions of functional target-derived fragments. This research presents evidence that a combination of duplication and inversion, as well as integration of exogenous DNA fragments and clustered interchromosomal rearrangements, can occur simultaneously. Furthermore, it was shown for the first time that the target-derived fragments were nevertheless functional despite these modifications, which may complicate mechanistic explanations. These results reveal a new example of unintended CRISPR/Cas9 editing events that can go unnoticed and have a significant impact on the conclusions drawn from experimental reads.
This clinical update looks at Graphite Bio's sickle cell disease candidate GPH101. GPH101 is an ex vivo CRISPR-edited cell therapy that is anticipated to provide a permanent cure by targeting the root cause of disease. Clinical trial enrolment is ongoing at multiple sites.
BigField GEG Tech's insight:
GPH101 is an ex vivo CRISPR-edited cell therapy designed to correct the HBB point mutation and should provide a permanent cure by targeting the root cause of the disease. Correction of the HBB gene results in a decrease in sickle cell hemoglobin production and restores normal expression of adult hemoglobin, thereby restoring normally functioning red blood cells. The candidate is engineered using patient-derived hematopoietic stem cells (HSCs) that are edited with Cas9-sgRNA gene editing machines and a DNA repair model, in a mechanism that essentially cuts the mutation from the genome and replaces it with the correct sequence. Cas9-sgRNA is delivered by ribonucleoprotein (RNP) complexes while delivery of a corrected DNA template is achieved by an adeno-associated virus 6 (AAV6) vector. The FDA approved an IND application in December 2020 for GPH101 and it will be evaluated in the Phase 1/2 CEDAR trial, an open-label, multicenter study to evaluate the safety, efficacy, and pharmacodynamics of the new therapeutic candidate in adults and adolescents with severe sickle cell disease. The trial will enroll approximately 15 adult and adolescent participants at up to five clinical trial sites in the United States.
Off-target genome editing in the liver can be reduced by using lipid nanoparticles to deliver oligonucleotides that disrupt the secondary structure of single-guide RNAs as well as short interfering RNAs targeting Cas9 mRNA.
BigField GEG Tech's insight:
In genome editing, it is crucial to ensure target-specific editing in diseased cells and to avoid altering the genome of spectator cells. Since a substantial fraction, i.e. 30-90%, of an intravenously injected dose of lipid nanoparticles (LPNs) accumulates in the liver, off-target editing in this organ must be minimized. According to a study published in Nature Biomedical Engineering , LNPs can be used to deliver two types of anti-CRISPR nucleic acid to the liver to disrupt genome editing in hepatocytes, thereby improving the specificity of CRISPR-Cas genome editing in the extrahepatic tissues and organs. To determine whether anti-CRISPR oligonucleotides could be used to reduce off-target editing in the liver, researchers first treated mice stably expressing Cas9 tagged with GFP with anti-CRISPR or scrambled oligonucleotides formulated in NLPs that preferentially target the liver. Two hours later, they treated the same mice with the same NPLs, but instead encapsulated gRNA targeting the GFP locus. Compared to scrambled oligonucleotides, the anti-CRISPR ones reduced the frequency of deletion mutations in hepatocytes by more than twofold. Therefore, Cas9-mediated on-target genome editing in extrahepatic tissues could be maintained while limiting off-target editing in the liver.
The ability to induce genome editing of the vascular endothelium has been challenging.
BigField GEG Tech's insight:
According to the journal Cell Reports, the lab of Youyang Zhao, PhD, of the Stanley Manne Children's Research Institute at Ann & Robert H. Lurie Children's Hospital in Chicago has developed a unique nanoparticle to deliver genome-editing technology such as CRISPR/Cas9 to endothelial cells. This is the first time that vascular endothelial cells can be reached for genome editing, as the usual way of delivering CRISPR/Cas9, via a virus, does not work for this cell type. With this nanoparticle, researchers can introduce genes to inhibit vascular damage and/or promote vascular repair, correct genetic mutations, and turn genes on or off to restore normal function. This technology also allows them to modify multiple genes at the same time. This is an important advance for the treatment of any disease caused by endothelial dysfunction.
Sonodynamic therapy uses ultrasound in combination with drugs to release harmful reactive oxygen species (ROS) at the site of a tumor.
BigField GEG Tech's insight:
Hepatocellular carcinoma, the most common form of liver cancer, and surgical treatment by removing part of the liver or transplanting a healthy liver is not suitable for patients with more advanced disease. That's why researchers have developed sonodynamic therapy (SDT), which uses ultrasound in combination with drugs to release harmful reactive oxygen species (ROS) at the site of a tumor. Because ultrasound can penetrate deep into tissue, SDT may be an effective, non-invasive way to treat hepatocellular carcinoma. However, the treatment is not very effective because cancer cells can quickly overcome the therapy by activating a gene called nuclear factor erythroid 2-related factor 2 (NFE2L2), which deploys cell detoxification and antioxidant enzymatic defenses.The researchers therefore used and encapsulated the CRISPR/Cas9 system and an ROS precursor molecule in lipid nanoparticles.Then, they treated hepatocellular carcinoma cells in a Petri dish with the nanoparticles. The lipid nanoparticles were taken up by the lysosomes of the cells. Ultrasound treatment caused the formation of ROS, which disrupted the lysosomes and allowed the CRISPR/Cas9 system to enter the nucleus and inhibit NFE2L2 gene expression. The ROS also damaged other cellular components. As a result, many more cancer cells died from SDT than without NFE2L2 gene editing. Next, the team injected the nanoparticle treatment into mice with implanted human hepatocellular carcinoma tumors. After 15 days of combined nanoparticle and ultrasound treatment, all of the mice's tumors disappeared and did not return.
BigField GEG Tech's insight:
Since CRISPR-Cas was first adapted for eukaryotic genome editing in 2013, the technology and the toolbox have advanced by leaps and bounds. Today, a number of CRISPR-based therapies are moving through clinical trials with promising results. Despite these improvements, a number of challenges remain in the field of CRISPR medicine, one of which is the challenge associated with the limited size capacity of adeno-associated viral (AAV) vectors used to deliver gene editing reagents to cells. Last year, the CasΦ family of CRISPR endonucleases (also known as Cas12j) was discovered as a very compact CRISPR system in ancient giant phages. In addition, CasΦ was shown to edit genomic DNA in mammalian and plant cells, but questions about its mechanism of action remained open. Thus, this discovery raised hopes that a solution to the AAV capacity challenge was at hand. Only a year later, Guillermo Montoya's team at the University of Copenhagen, Denmark, answered these questions by solving, using Cryo-em, the structure of Cas12j3 in complex with a DNA target after cleavage, paving the way for future optimization of CasΦ genome editors and facilitating the future redesign of this CRISPR system for therapeutic genome editing.
Moving beyond viral vectors and lipid nanoparticles, Spotlight is conjugating Cas proteins to agents that will home endonucleases and their guide RNAs to targets in vivo.
BigField GEG Tech's insight:
Spotlight Therapeutics wants to reimagine CRISPR delivery. The company's three co-founders focused on delivery as an area of CRISPR that needed new thinking. The central idea is to target the Cas endonuclease and its gRNA to specific cell types in vivo using a 'component library' of cell-penetrating peptides (CPPs), antibodies and ligands. These components can be recombined into a selection of targeted active gene editors (TAGEs). TAGEs combine a cell-targeting antibody with CPPs bound to a Cas endonuclease pre-loaded with a gRNA of interest. They search for the target cells, cross the cell membrane and enter the nucleus, where they can modify the locus of interest. To identify targeting molecules that can be used in TAGEs, Spotlight uses a combination of rational design and high-throughput screening. The company claims that its discovery pipeline allows it to identify targeting molecules that work in practice, not just in theory. Their screening platform ensures that a given molecule effectively delivers the editors to the nucleus. One of the advantages of ex vivo therapies is, of course, the ability to perform quality control on the modified cells.
|
The landmark decision could transform the treatment of sickle-cell disease and beta-thalassaemia — but the technology is expensive.
BigField GEG Tech's insight:
The landmark decision could transform the treatment of sickle-cell disease and beta-thalassaemia — but the technology is expensive.... and safe.....?
Simple, efficient and well-tolerated delivery of CRISPR genome editing systems into primary cells remains a major challenge. Here we describe an engineered Peptide-Assisted Genome Editing (PAGE) CRISPR–Cas system for rapid and robust editing of primary cells with minimal toxicity. The PAGE system requires only a 30-min incubation with a cell-penetrating Cas9 or Cas12a and a cell-penetrating endosomal escape peptide to achieve robust single and multiplex genome editing. Unlike electroporation-based methods, PAGE gene editing has low cellular toxicity and shows no significant transcriptional perturbation. We demonstrate rapid and efficient editing of primary cells, including human and mouse T cells, as well as human hematopoietic progenitor cells, with editing efficiencies upwards of 98%. PAGE provides a broadly generalizable platform for next-generation genome engineering in primary cells. Peptide-assisted genome editing enables efficient single and multiplex editing in hematopoietic cells.
BigField GEG Tech's insight:
Current methods of getting CRISPR-Cas systems into cells, which include the use of carrier viruses and electric pulses, are inefficient for cells taken directly from patients, called primary cells. These methods also typically kill many of the cells they are used on, and can even cause broad unwanted changes in gene activity. In a new study, which appeared in Nature Biotechnology, researchers explored the use of small, virus-derived protein fragments, called peptides, to pilot CRISPR-Cas molecules more efficiently through the outer membranes of primary human cells and into their nuclei. Notably, researchers found that a fused combination of two modified peptides : one found in HIV and one in influenza viruses, could be mixed with CRISPR-Cas molecules to get them into primary human or mouse cells and their nuclei with efficiencies of up to nearly 100 percent, depending on the cell type and with almost no toxicity or gene-expression changes. The team demonstrated the approach, which they call peptide-assisted genome editing (PAGE), for several types of envisioned cell therapy including CAR T cell therapies. The scientists expect the new technique to be particularly useful for modifying T cells and other cells from a patient's own body to make cell therapies.
A team of researchers at Northwestern University has devised a new platform for gene editing that could inform the future application of a near-limitless library of CRISPR-based therapeutics.
BigField GEG Tech's insight:
A team of researchers at Northwestern University has designed a new platform for gene editing. Using chemical design and synthesis, the team brought together CRISPR-Cas9 with a therapeutic technology born in their lab to overcome a critical limitation of the gene editing tool: its delivery. The team developed a way to transform the Cas-9 protein into a spherical nucleic acid (SNA) and load it with critical components as needed to access a wide range of tissue and cell types, as well as the intracellular compartments needed for gene editing. This study shows how CRISPR SNAs can be delivered across the cell membrane and into the nucleus while maintaining bioactivity and gene editing capabilities. The researchers used Cas9, a protein required for gene editing, as the core of the construct, and attached DNA strands to its surface to create a new type of SNA. In addition, these SNAs were preloaded with RNA capable of gene editing and fused with peptides to control their ability to navigate the compartmental barriers of the cell, maximizing efficiency.
A Penn State-led team of interdisciplinary researchers has developed techniques to improve the efficiency of CRISPR-Cas9, the genome editing technique that earned the Nobel Prize in 2020.
BigField GEG Tech's insight:
Researchers have developed a more efficient and accessible process for applying CRISPR-Cas9 systems in human pluripotent stem cells (hPSCs) that could advance diagnoses and treatments for genetic disorders. To improve this system, the researchers changed the way the tool is delivered to stem cells, using modified RNA (modRNA). ModRNA differs from plasmid DNA in that it replaces one of the basic substrates found in RNA with a chemically modified version, and it is stabilized by a stronger structural support. About 90% of the cells received the modRNA from a single transfection. The researchers also found that the length of time the modRNA was in place was ideal: long enough to modify the cells, but not too long to cause off-target activity. However, when the Cas9 modRNA is successfully delivered to the target gene, it creates a double-stranded break in the genome, which some cells will try to repair and pass the repair, or "mutation," to their offspring. To reduce the toxic side effects of Cas9 and help the modified cells survive, the researchers also introduced a small protein known to help cells grow. This added protein inhibited cell death and improved the efficiency of Cas9 editing by up to 84%.
DNA-based information is a new interdisciplinary field linking information technology and biotechnology.
BigField GEG Tech's insight:
Despite DNA's promise of high stability, high storage density and low maintenance cost, researchers face problems in accurately rewriting the digital information encoded in DNA sequences. To solve the rewriting problem, the researchers established an in vivo double plasmid system using a rationally designed coding algorithm and an information editing tool. This dual-plasmid system is suitable for storing, reading, and rewriting various types of information, including text, codebooks and images. It fully explores the encoding capability of DNA sequences without the need for addressing indices or backup sequences. It is also compatible with various types of coding algorithms, allowing high coding efficiency. For example, the coding efficiency of the current system reaches 4.0 bits per nucleotide. To achieve high efficiency and reliability in rewriting complex information stored in exogenous DNA sequences in vivo, a variety of CRISPR-associated proteins (Cas) and recombinases were used. The information rewriting tool thus became highly adaptable to complex information, resulting in a rewriting reliability of up to 94%, which is comparable to existing gene editing systems.
Wake Forest Institute for Regenerative Medicine (WFIRM) scientists working on CRISPR/Cas9-mediated gene editing technology have developed a method to increase efficiency of editing while minimizing DNA deletion sizes, a key step toward developing gene editing therapies to treat genetic diseases.
BigField GEG Tech's insight:
Although CRISPR/Cas9 mainly generates short insertions or deletions at the target site, it can also make large deletions of DNA around the specific target site. These large deletions pose safety concerns and can reduce the efficiency of functional editing. The WFIRM team is looking at ways to reduce the risk of this happening. The research described in their recent paper, published recently in Nucleic Acids Research, aimed to combat the generation of unpredictable long DNA deletions on target and find a way to prevent this, a key step towards the development of gene editing therapies to treat genetic diseases. The team evaluated a variety of human cells and genes of interest and found that fusing DNA polymerase I or the Klenow fragment to the Cas9 enzyme minimised large, unanticipated deletions of genomic DNA without sacrificing the efficiency of genome editing. On the contrary, it even increased editing efficiency in primary human cells.
Most pharmaceuticals don't reach the brain, leaving cognitive impairments due to diseases like mucopolysaccharidoses (MPS) hard to treat. Now, a Brazilian research team has developed a method for nasal delivery of CRISPR reagents to the brain that improves MPS cognitive symptoms in mice. Experiments will proceed with monkeys later this year.
BigField GEG Tech's insight:
Mucopolysaccharidosis I is caused by mutations in the gene for the enzyme alpha-L-iduronidase (IDUA). This enzyme is required for the breakdown of substances called glycosaminoglycans (GAGs) that are by-products of chemical reactions in the body's cells. In 2018, a team of researchers was able to demonstrate the feasibility of CRISPR gene editing in vivo in mice via intravenous injection. The treatment partially restored IDUA enzyme activity in organs such as the heart, lungs, liver and kidneys, but not in the brain because the blood-brain barrier protects it. However, this is a problem for patients with the severe form of the disease who may develop cognitive decline. The researchers therefore had the idea of going through the nose to reach the olfactory bulb just above. As a result of the experiments, it was finally shown that non-invasive administration of CRISPR reagents via nasal administration allows gene editing and partial restoration of IDUA activity in the brain and improved cognitive function, while biomarkers of the disease in other organs also improved.
Intellia Therapeutics recently announced that the first patient had been dosed in its Phase 1/2a CRISPR trial for acute myleoid leukaemia (AML). The new therapeutic candidate, NTLA-5001, is a CRISPR-edited T cell receptor therapy designed to targeted Wilm's Tumour (WT1) antigen, which is found on AML and several other blood cancers as wel
BigField GEG Tech's insight:
Intellia Therapeutics recently announced that the first patient has received a dose in its Phase 1/2a CRISPR trial for acute myeloid leukemia (AML). The new therapeutic candidate, NTLA-5001, is a CRISPR-edited T-cell receptor therapy designed to target Wilm's tumor antigen (WT1), which is found on AML and several other blood cancers as well as certain types of solid cancer. To generate NTLA-5001, T cells are isolated from a patient with AML and the T cell receptor (TCR) is replaced with a naturally occurring high avidity TCR (i.e. from a healthy donor) with specificity for WT1. Complete elimination of the endogenous TCR is achieved by CRISPR-Cas9-mediated replacement of the TRAC locus with the WT1 TCR, and subsequent site-specific inactivation of the TRBC locus, which encodes the T cell receptor beta chains. For TCR locus disruption, SpCas9 and gRNAs are delivered to cells via lipid nanoparticles. The WT1 TCR is delivered to cells via adeno-associated virus (AAV) transduction. NTLA-5001 is therefore being evaluated in a multi-center, single-dose Phase 1/2a study that will assess its safety, tolerability, cellular kinetics (CK), activity and pharmacodynamics (PD) in AML participants.
Researchers at Texas Biomedical Research Institute in San Antonio, Texas have developed a novel strategy to inhibit human immunodeficiency virus (HIV) replication, using guide RNAs that target highly conserved regions of HIV-1 in conjunction with the CRISPR-Cas13d nuclease system.
BigField GEG Tech's insight:
A recent publication in Viruses detailed a CRISPR-based strategy to effectively target and control HIV-1 infections in vitro with CasRx. Dr. Smita Kulkarni's laboratory at the Texas Biomedical Research Institute successfully combined a novel set of guide RNAs (gRNAs), known as polyHIV, with CasRx to target and inhibit HIV-1 replication. This work demonstrates for the first time the potential of the CRISPR-Cas13d nuclease system to target acute and latent HIV infections that could potentially be used to improve the effectiveness of current HIV treatment options. Kulkarni's team strategically selected four sites in the HIV genome that are more than 70% conserved from the HIV-1 transcript sequences deposited in the LANL database and specific gRNAs were designed against these sites. The four gRNAs were selected from 20 candidates to form a poly-gRNA assembly (polyHIV) and paired with CasRx, a Cas protein derived from the Cas13d family with high RNA cleavage activity. The selected target sites were non-overlapping and located in the gag, pol, protease (prot), integrase (int), cPPT and central termination sequence (CTS) coding regions in the HIV-1 genome. The study is the first to provide a single vector with four gRNAs targeting non-overlapping conserved regions of HIV-1.
Adeno-associated virus (AAV) vectors are important delivery platforms for therapeutic genome editing but are severely constrained by cargo limits. Simultaneous delivery of multiple vectors can limit dose and efficacy and increase safety risks. Here, we describe single-vector, ~4.8-kb AAV platforms that express Nme2Cas9 and either two sgRNAs for segmental deletions, or a single sgRNA with a homology-directed repair (HDR) template. We also use anti-CRISPR proteins to enable production of vectors that self-inactivate via Nme2Cas9 cleavage. We further introduce a nanopore-based sequencing platform that is designed to profile rAAV genomes and serves as a quality control measure for vector homogeneity. We demonstrate that these platforms can effectively treat two disease models [type I hereditary tyrosinemia (HT-I) and mucopolysaccharidosis type I (MPS-I)] in mice by HDR-based correction of the disease allele. These results will enable the engineering of single-vector AAVs that can achieve diverse therapeutic genome editing outcomes. Long-term expression of Cas9 following precision genome editing in vivo may lead to undesirable consequences. Here we show that a single-vector, self-inactivating AAV system containing Cas9 nuclease, guide, and DNA donor can use homology-directed repair to correct disease mutations in vivo.
BigField GEG Tech's insight:
Most studies using AAVs to deliver gene therapy attempt to insert a Cas9 nuclease and a single guide RNA (sgRNA), along with their respective promoters, into the vector to create a single double-stranded break in the DNA. Given the 4.7 kb packaging limit of most AAVs, this is typically a significant challenge, especially for studies aimed at correcting genetic mutations by providing a donor DNA template for HDR. In this study, members of Professor Erik Sontheimer's lab at the University of Massachusetts Chan Medical School demonstrated the use of a cleverly designed, self-inactivating AAV vector with a compact Cas9 variant for therapeutic CRISPR genome editing in vivo. The study takes this packaging problem a step further, with the goal of creating a vector with enough cargo space to hold two sgRNAs rather than just one. In doing so, CRISPR could be used to excise pathogenic trinucleotide repeat expansions, such as that which causes Friedreich's ataxia. With the compact size of Staphylococcus aureus Cas9 (Nme1Cas9), this makes it easier to package into AAVs. It has a dinucleotide PAM sequence, which means it has a wider range of genomic targets and it displays high editing efficiencies in mammalian cells with low off-target activity, making it ideal for therapeutic applications.
This up-to-date roundup presents the 7 clinical-stage gene-editing approaches to sickle cell disease, which affects millions of people worldwide and is the most common inherited blood disorder in the United States. Current clinical-stage approaches to treating this disease include CRISPR-Cas9, CRISPR-Cas12a, and base editing.
BigField GEG Tech's insight:
Sickle cell disease (SCD) is a group of diseases characterized by defective adult hemoglobin, and it belongs to a larger family of autosomal recessive blood disorders known as hemoglobinopathies. A number of treatments exist to manage the symptoms and complications of sickle cell disease, but none of them are curative. Hydroxyurea was the first drug to receive FDA approval for SCD in 1997. It is an anticancer agent that promotes fetal hemoglobin production by a poorly understood mechanism and is widely used today despite side effects. Recently approved treatments include Endari, which increases L-glutamine levels in the blood, triggering a cascade of events that reduces oxidative stress in sickle cells, allowing them to retain some function, Oxbryta, which prevents defective hemoglobin from aggregating, improving SCD symptoms, and Adakveo, a monoclonal antibody that blocks P-selectin, decreasing SCD-related inflammation. Gene editing offers new hope for patients with sickle cell disease. There are currently seven clinical-stage candidates in development, five of which aim to restore fetal hemoglobin (HbF) expression through gene editing of patient-derived cells, while the other two aim to address the root of the disease by directly repairing the underlying disease mutation.
BigField GEG Tech's insight:
Editas Medicine said today its lead candidate EDIT-101, an in vivo CRISPR gene editing treatment for Leber congenital amaurosis-10 (LCA10), showed positive initial clinical data showing it to be safe, and to have generated "signals" of efficacy in two of three patients in the study’s adult mid-dose cohort. |


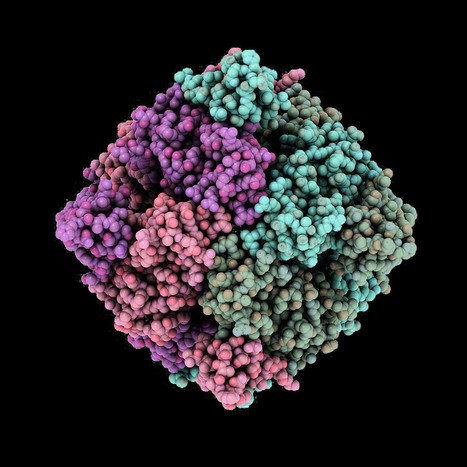


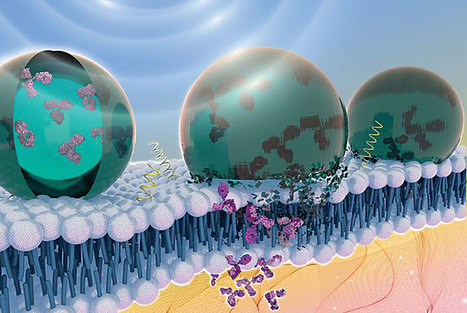




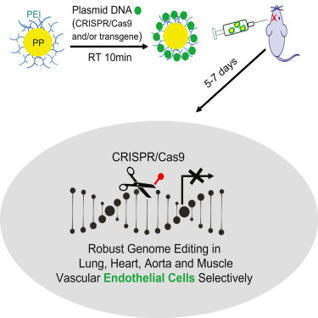


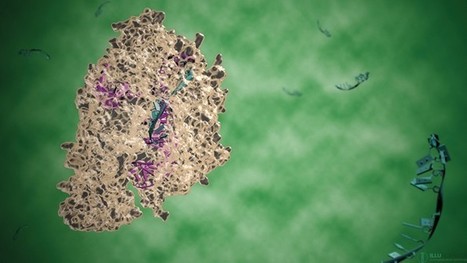






All-in-one viral particles have been developed through in-depth engineering of each major component. They can contain all the necessary components: prime editor proteins, prime editing guide RNAs, and nicking single guide RNAs. The engineered virus-like particles (eVLP) architectures that facilitate cargo release and localization have also been optimized. Prime editor engineered virus-like particles (PE-eVLPs) v3 and v3b function as transient ribonucleoprotein complexes and show a staggering 65- to 170-fold increase in editing efficiency in human cells compared with previous master editor constructs. In two mouse models of genetic blindness, a single injection of PE-eVLP v3 resulted in significant editing, restoration of protein expression and partial recovery of visual function. Optimized PE-eVLPs offer enhanced safety by minimizing off-target editing and eliminating the risk of integrating oncogenic transgenes during in vivo administration of core editor ribonucleoproteins.
GEG Tech has designed its own All-In-One plateforme enabling to efficiently deliver Cas9 and RNA in the same particle and gives acces to its technology through different partnerships.